Bonds are an important part of a wide range of products, from EV batteries to aerospace components. To ensure the products’ safety and long-term quality, these bonds must be reliable and durable. Especially when they are exposed to stresses, loads, and high levels of energy.
To achieve this, strong bonds are needed.
Cleaning a surface before joining different materials is an excellent way to generate stronger bonds. This is one of the subjects we will explain in detail in this article.
But first, we will go over the key theory around bond strength.
Table of Contents
- What is Bond Strength?
- What is a Chemical Bond?
- What is a Mechanical Bond?
- How is Bond Strength Measured?
- Surface Preparation to Improve Bond Strength
What is Bond Strength?
The term bond strength refers to the force holding different materials or atoms together. It measures the amount of energy needed to break different types of bonds, such as chemical bonds and adhesive bonds. The stronger the bond, the more energy is required to break it.
What is a Chemical Bond?
When two surfaces bond together, powerful chemical forces are at stake. Atoms form what we call chemical bonds with one another.
Chemical bonds are electrostatic forces that join atoms together to form molecules like water and oxygen. Chemical bonds also act as a natural force that can join different surfaces together.
Why Atoms Form Bonds
Atoms form chemical bonds because they seek to achieve balance through a neutral electric charge. They behave a bit like magnets: similar charges repel one another while opposite charges attract one another. Likewise, atoms that have a positive charge want to bond with atoms that have a negative charge.
The Role of Electric Charges in Bonding
Atoms consist of protons, neutrons, and electrons that each have different electric charges. Protons have a positive electric charge, neutrons have no electric charge, and electrons have a negative electric charge. The difference of protons and electrons in an atom determines its electric charge.
As a result, atoms with a positive electric charge (cations) are attracted to atoms with a negative electric charge (anions). Atoms can achieve electrical stability by joining together.
While the number of protons in an atom cannot change, the number of electrons can. Chemical bonds are created through the interaction of electrons between different atoms, leading to the formation of stable compounds.
The 3 Types of Chemical Bonds
Three types of chemical bonds can be formed to achieve electrical stability. These include covalent bonds, ionic bonds, and metallic bonds. When joining different surfaces together, only covalent bonds and ionic bonds are relevant.
|
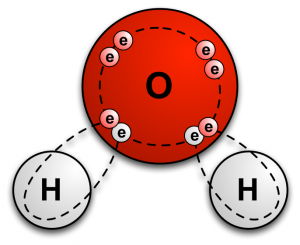
A covalent bond is created when atoms share electrons with one another. This sharing of electrons allows each atom to fill its outer shell of electrons (called the valence shell). It is a chemical bond that almost exclusively happens with nonmetals. For example, a water molecule (H2O) consists of one oxygen atom and two hydrogen atoms that are held together by covalent bonds. The covalent bonds are as follows: the oxygen atom shares two of its electrons (one with each hydrogen atom), and each hydrogen atom shares its single electron with the oxygen atom. In total, 4 electrons are shared. |
The covalent bonds that join oxygen and hydrogen atoms to form water molecules. Image courtesy of ErrantScience.
|
|
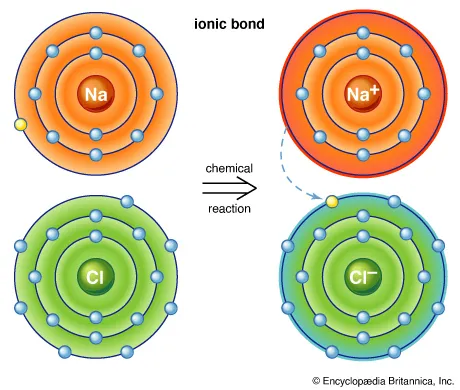
An ionic bond is created when electrons are transferred from one atom to another. This swapping of electrons typically occurs between metals and nonmetals. In this exchange, metals typically lose electrons while nonmetals gain electrons. Positively charged atoms are attracted to negatively charged atoms, creating electrostatic attraction that bonds them together. For example, table salt is formed by ionic bonding. Sodium (a metal) transfers an electron to chlorine (a nonmetal), resulting in a positively charged sodium ion (Na+) and a negatively charged chlorine ion (Cl−). With opposite electric charges, the two atoms are attracted to one another and form a stable ionic compound. |
The ionic bond that joins sodium (Na) and chlorine (Cl) atoms to form common table salt. Image courtesy of Encyclopædia Britannica.
|
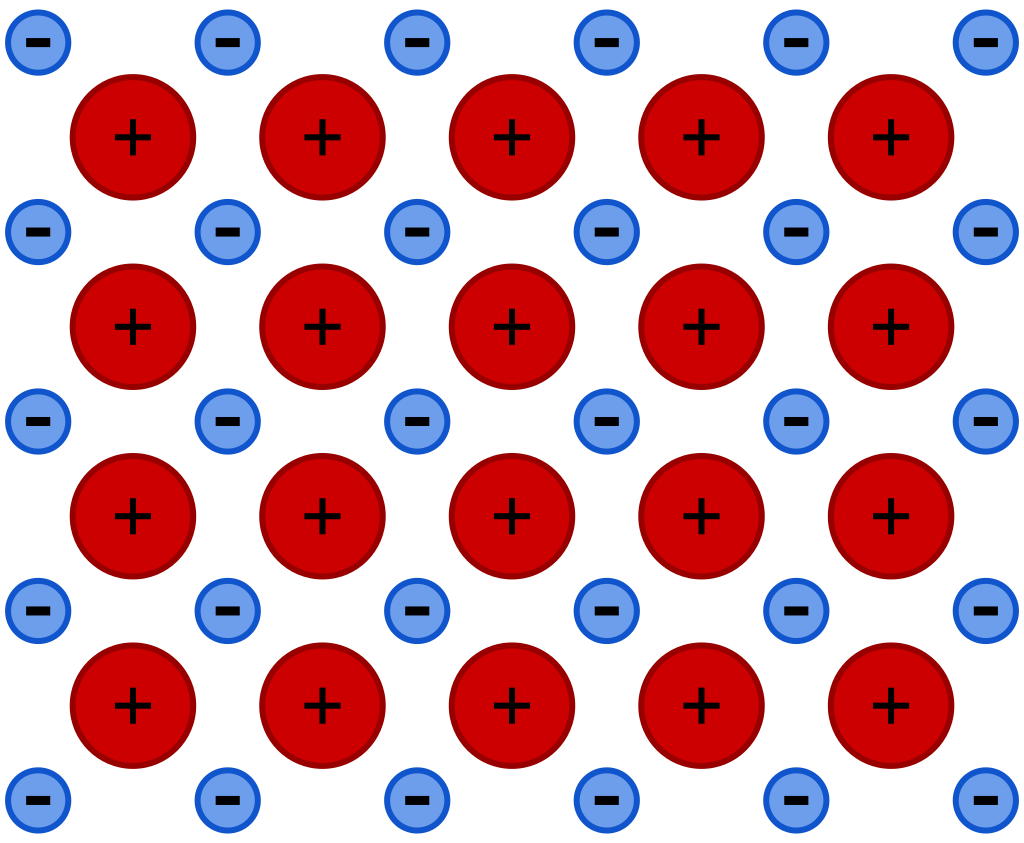
| A metallic bond is when multiple atoms of the same metal (such as copper, iron, or aluminum) share electrons together in a metallic lattice. The metallic lattice is commonly described as a sea of free-floating electrons, as the electrons are not tied to atoms. Because metal atoms lose electrons to the metallic lattice, they become positively charged. This creates an electrostatic force that bonds the metal atoms to the metallic lattice, which is negatively charged. |
The sea of free-floating electrons in metallic bonds. Image source is Wikipedia.
|
What is a Mechanical Bond?
Unlike chemical bonds, mechanical bonds do not involve electrons. A mechanical bond refers to the physical entanglement of different components together. It is widely used to join dissimilar materials. Mechanical fasteners (such as screws, bolts and rivets) and adhesives are common tools used to create mechanical bonds.
Friction between different surfaces is another way to create mechanical bonds (called mechanical interlocks). Manufacturers use different means to increase friction between bonded surfaces, such as grit blasting, laser texturing, and chemical etching. Friction allows adhesives to mechanically interlock with surface irregularities and improve their bond strength.
More advanced mechanical bonding is also researched at the molecular level, where different molecules are threaded through one another to create a bond that physically interlocks them. Examples of mechanically bonded molecules include rotaxanes and catenanes, but their practical implementation in real-world applications is still ongoing research and development.
How Is Bond Strength Measured?
Chemical Bond Measurements
The energy required to break a chemical bond is measured in kilojoules per mole (kJ/mol). It is called the bond dissociation energy (for a single bond) or the bond energy (for multiple bonds).
For example, the energy required to break a single H-O bond in water is ≈492 kJ/mol. Since a water molecule has two H-O bonds, ≈984 kJ/mol is needed to break a single water molecule.
It’s important to note that when multiple water molecules are joined together, hydrogen bonds are formed between them. These bonds are weak and require low energy to break, but they are important to consider when trying to break a volume of water.
Whenever a chemical bond is broken, the energy that held it together is released. This energy release is called an enthalpy change, and it is essential to understand thermodynamic reactions (i.e., how much heat is released in chemical reactions).
Mechanical Bond Measurements
|
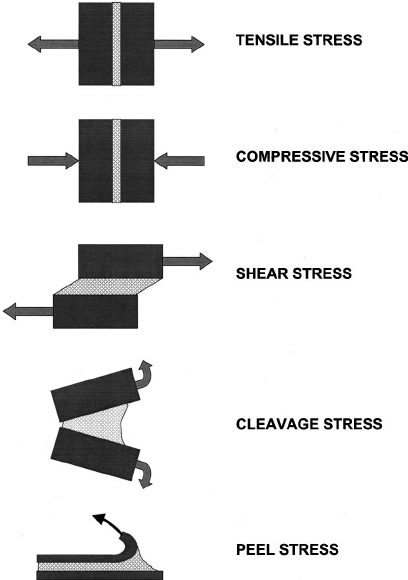
The energy required to break a mechanical bond is typically measured in megapascals, a unit of pressure that expresses the number of newtons per square millimeter (force per area). Megapascals (MPa) can measure the strength of adhesive bonds or of other types of mechanical bonds. Mechanical bond measurements can help gauge the performance of materials in practical applications, ensuring that they are strong enough to withstand the conditions to which they will be exposed. Here are common characteristics used to measure the strength of mechanical bonds:
|
5 types of stresses that can cause mechanical bonds to break. Image from Adhesive bonding in microelectronics and photonics by Yacobi et al. |
The strength of mechanical bonds can be tested for other characteristics as well, such as cleavage strength and compressive strength. The company 3M made great animations of each type of stress here.
Surface Preparation to Improve Bond Strength
Surface preparation is essential to maximize the strength of the bond between two surfaces. Bond strength can be improved by removing contaminants, roughening the surface, and modifying the chemical composition of the surface. Several methods can be used to prepare surfaces for bonding, including laser cleaning, mechanical abrasion, chemical cleaning, and plasma treatments.
Let’s look at how each aspect of surface preparation improves bonding:
- Contaminants (such as oxides, dust, greases, and oils) need to be removed because they interfere with bonding. They create a barrier between the adhesive and the surface, cause unwanted chemical reactions, and diminish the energy available for adhesive forces between molecules.
- Rough surfaces increase the surface area available for bonding and improve mechanical interlocking through peaks and valleys.
- The chemical composition of the surface can be modified to create favorable conditions for chemical bonds and intermolecular forces (such as hydrogen bonds and van der Waals forces).
To see how different methods improve bond strength, we studied the effect of laser texturing and sandblasting on aluminum joints bonded using Loctite EA 9460. Let’s look at the results of the tests.
Pull-Off Strength & Shear Strength
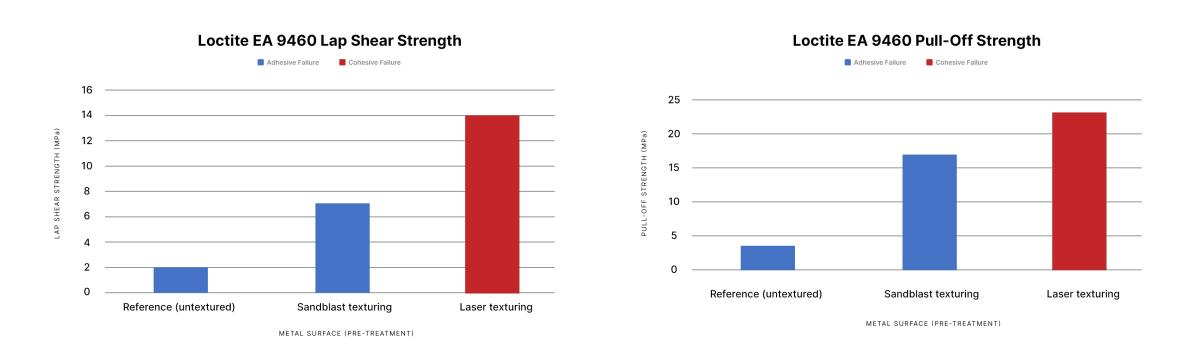
Results show that laser texturing provides stronger bonds than sandblasting, outperforming it both in terms of lap shear strength and pull-off strength.
- The lap shear strength with laser texturing was 14 MPa compared to 7 MPa for sandblasting.
- The pull-off strength with laser texturing was 23 MPa compared to 17 MPa for sandblasting.
Adhesive Failure vs. Cohesive Failure

Image Courtesy of Tom Brown
If you apply enough force, bonds will fail eventually. The way in which a bond fails shows its weakest point.
- An adhesive failure is when the bond between the adhesive and the substrate fails.
- A cohesive failure is when the bond within the adhesive fails.
If a cohesive failure happens, it means that the adhesive bond is strong enough, as there is a weaker point within the adhesive that will break before.
During our tests:
- Surface preparation with laser texturing resulted in cohesive failure. This means that the bond with the substrate was stronger than the bond within the adhesive.
- Surface preparation with sandblasting resulted in adhesive failure. This means that the bond between the substrate and the adhesive is weaker.
This shows that laser texturing provides a real improvement over sandblasting.
Reach Out to Discuss How to Improve Bond Strength
At Laserax, our experts can guide you to improve bond strength with laser technology. If you’re dealing with coatings or adhesives, reach out to discuss your needs.